Beyond Compliance: Why Your Competitor’s Failures Are Your Most Valuable Early Warning System
Leveraging MAUDE adverse event data for competitive insights, early risk detection, design improvements, and regulatory readiness

Axion

Every senior leader in the MedTech industry faces the same challenge: how to deliver world-class quality while navigating an increasingly complex regulatory landscape. The conventional wisdom is simple - focus on meeting internal quality metrics, track your own compliance rates, and respond efficiently to regulatory queries.
However, the mindset drives quality teams to focus on reacting to the regulator’s next move. The most effective quality and risk management is driven not by reacting to your own failures, but by proactively incorporating external market signals into your design and risk management processes. We call this the shift from Reactive Compliance Mode to Proactive Design Mode.
Leveraging Regulatory Data for Product Intelligence
For a VP of Quality / Regulatory Affairs, time is the scarcest resource. Quality engineers are often pulled into Requests for Additional Information (RFAIs), spending days to weeks reviewing every complaint incident for a failure mode. This time spent compiling a retroactive response to an FDA query is time diverted from designing the next generation of safe, effective devices.
Imagine this: You launched a new infusion pump about 9 months ago. Your internal complaint rate is low and stable, and customers are delighted with product performance. Then, a regulatory body issues a query about leaks. Why? Because they see signals through a wider lens – your competitor’s pumps have been recently spiking in Medical Device Reports (MDRs) for major leakages that your team wasn’t tracking.
The FDA’s Manufacturer and User Facility Device Experience (MAUDE) database holds millions of user reports, capturing failure modes and issues that your specific device may not yet have experienced. The regulator is already comparing your performance to the entire industry; doing the same will allow you to anticipate questions, identify emerging risks earlier, and take action accordingly.
The status quo views MAUDE as a historical reference. Axion’s perspective challenges this: While some manufacturers look internally for early warning signs of potential failure modes, best-in-class organizations leverage MAUDE as a proactive design tool. It allows leaders to set baselines for future product quality and proactively mitigate anticipated risks before the first device is even implanted. We’ve realized that the most innovative MedTech companies treat external data - specifically Medical Device Reports (MDRs), competitive device data, and regulatory query trends - not as a compliance burden, but as a free and powerful early warning system for product design. A failure mode that is trending in your competitor’s MDRs today is highly likely to appear in your product tomorrow.
Additionally, comparing the sheer volume of adverse events across competitive devices will help Quality leaders gain crucial context. If your competitor has 20 MDRs and you have zero, try shifting your perspective from reactive, “Why are we doing so well?” to proactive curiosity, “What risk factor are we currently overlooking, and why is the regulator asking them first?” If a signal appears in your competitor's devices, regulators will look to understand its relevance for your product as well.
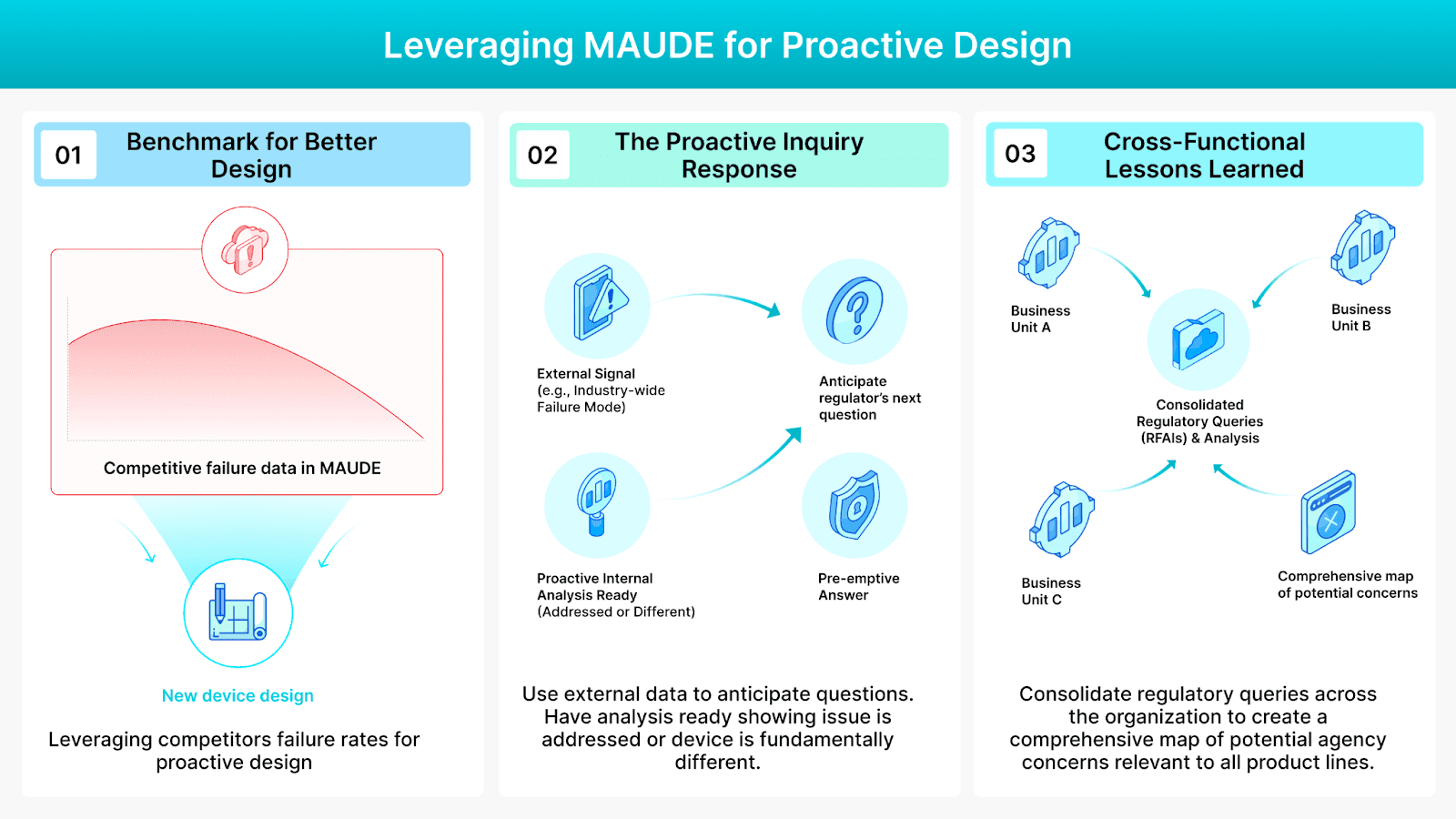
Three Ways to Leverage Regulatory Data For Your Advantage
Benchmarking for Better Design: Before finalizing the requirements for a new device, utilize competitive MAUDE data to establish a target baseline. If a competing product has a 20% failure rate for a specific failure type, you can now target something better. External failure rates become your minimum viable quality threshold.
The Proactive Inquiry Response: Use external signals to anticipate the regulator’s next question. When an agency starts looking into a specific failure mode across the industry (e.g., component migration), you should already have the internal analysis ready showing how you have proactively addressed it.
Cross-Organizational Lessons Learned: The same issues that lead to an RFAI on one of your product lines are often relevant to others. Consolidating and analyzing all regulatory queries across the organization, even across different Business Units, creates a comprehensive map of potential agency concerns and anticipated areas where they’ll likely deep dive.
How to Shift from Reactive Compliance to Proactive Design
Moving from reactive compliance to proactive design requires a shift in data strategy, not just a new tool. Here are three steps to begin leveraging external signals to your advantage:
Assign Ownership: Designate a specific quality or regulatory analyst to be the "Market Signal Miner" - someone tasked with regularly compiling and disseminating competitive analysis and industry-wide regulatory trends to the relevant product engineering teams.
Integrate External Benchmarks: Encourage New Product Development (NPD) project charters to include a section that explicitly states a "Competitive Adverse Event Baseline" derived from public data, making it a non-negotiable quality target.
Run a "Failure Simulation": For your top-selling device, simulate a failure mode currently trending in your competitor's MDRs. Run this through your internal complaint and CAPA process to see if your current system would successfully flag the issue and take action, even though you haven't seen it yet in your device.
By broadening your data sources to include external early signals such as MDRs and regulatory inquiry patterns, you stop treating quality as a reporting requirement and start treating it as a strategic, proactive advantage. With Axion, best-in-class Quality teams proactively monitor signals across MAUDE and other external sources to benchmark performance against peers, understand top reported failure modes across competitors, and take action before the regulators knock on their door.
To explore how this Proactive Design Framework empowered by Axion AI applies to your specific context, our next conversation is the best place to start. Contact us about how we can help you leverage competitive intelligence to your advantage.
Share


